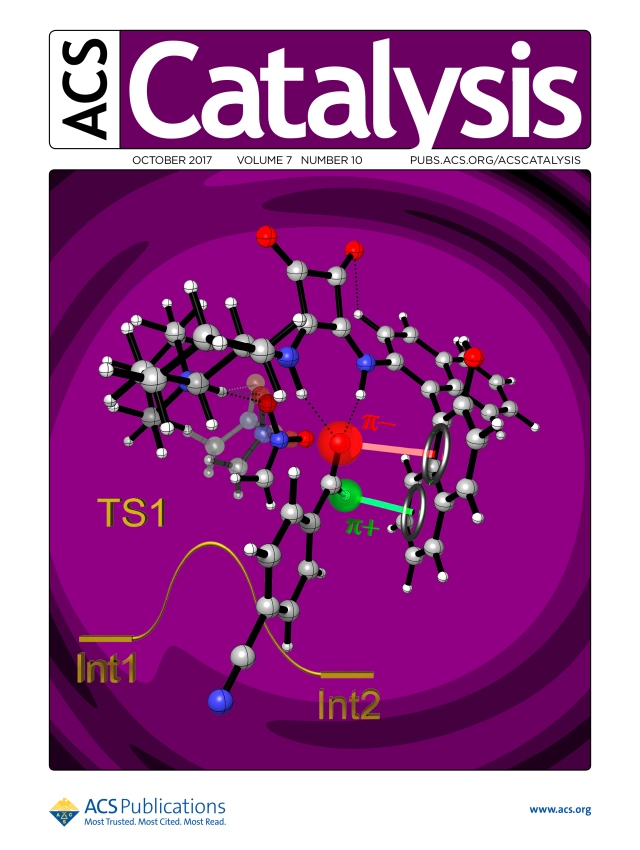
Our Asymmetric Organocatalysis (H-OCA) research group has developed a new enantioselective process for carrying out the Henry reaction. This reaction produces β-nitro alcohols, a group of compounds of highest interest, as they are frequently found in the structure of certain biologically active compounds, such as (S)-Propanolol and (S)-Moprolol -both are drugs commonly used in the treatment of hypertension-.
The importance of this method lies chiefly in its catalyst: a new squaramide that has demonstrated a trifunctional activation of substrates. Thus, it is possible reducing the catalyst loading up to 0.25 mol%. This is a very low proportion as compared to currently used in organocatalytic reactions, which use up to 10-20 mol% of catalyst.
Recently, our enantioselective process has been the focus of attention of Synfacts, a top journal devoted to discuss current most significant results published in international high rank scientific journals that are selected by their singular interest to the scientific communities in different Chemistry fields. This reinforces the significance of the method developed by the H-OCA, which is expected to have a broad impact in current and future scientific developments.
Trifunctional Squaramide Catalyzed Enantioselective Henry Reaction. Alegre-Requena, V.; Marqués-López, E.; Herrera, R. P. Adv. Synth. Catal. 2016, 358, 1801–1809. Highlighted in List, B; Schreyer, L. Synfacts 2016, 12, 743.